Section 3 4 Continued Elements and Compounds
- Slides: 13
Download presentation
Section 4: Elements and Compounds A compound is a combination of two or more elements. K What I Know W What I Want to Find Out L What I Learned

Essential Questions • What distinguishes elements from compounds? • How is the periodic table organized? • What are the laws of definite and multiple proportions and why are they important? Copyright © Mc. Graw-Hill Education Elements and Compounds
Vocabulary Review New • proportion • • • Copyright © Mc. Graw-Hill Education element periodic table compound law of definite proportions percent by mass law of multiple proportions Elements and Compounds

Elements • An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. • 92 elements occur naturally on Earth. • Each element has a unique name and a one, two, or three-letter symbol. Copyright © Mc. Graw-Hill Education Elements and Compounds
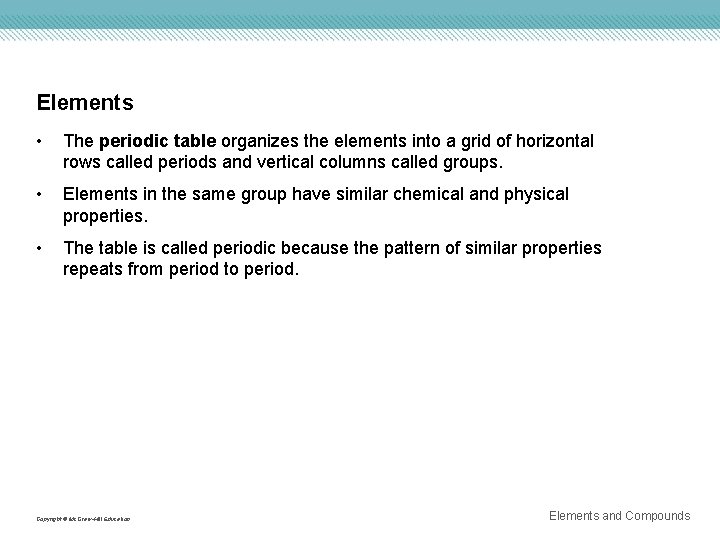
Elements • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. • Elements in the same group have similar chemical and physical properties. • The table is called periodic because the pattern of similar properties repeats from period to period. Copyright © Mc. Graw-Hill Education Elements and Compounds
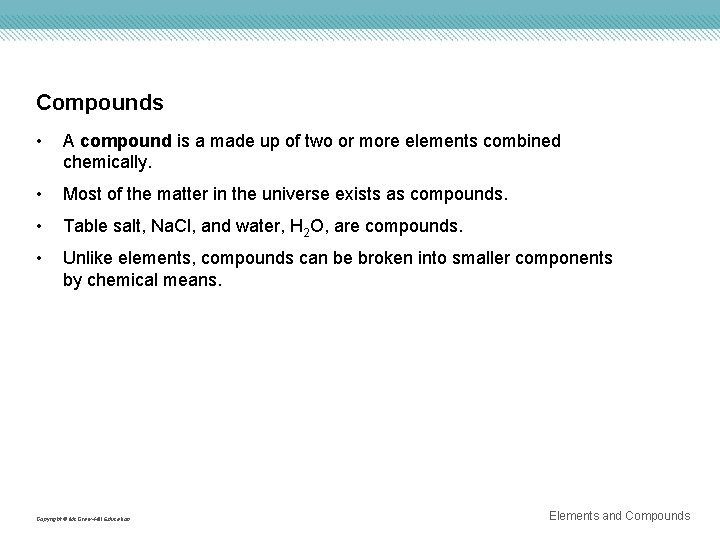
Compounds • A compound is a made up of two or more elements combined chemically. • Most of the matter in the universe exists as compounds. • Table salt, Na. Cl, and water, H 2 O, are compounds. • Unlike elements, compounds can be broken into smaller components by chemical means. Copyright © Mc. Graw-Hill Education Elements and Compounds
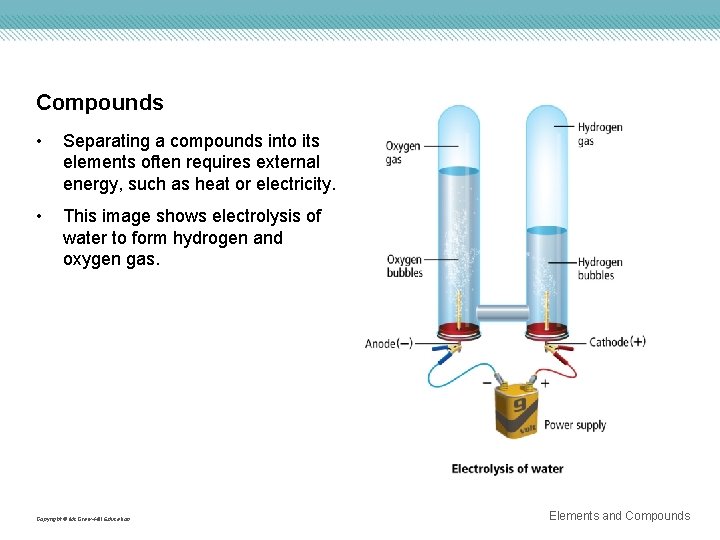
Compounds • Separating a compounds into its elements often requires external energy, such as heat or electricity. • This image shows electrolysis of water to form hydrogen and oxygen gas. Copyright © Mc. Graw-Hill Education Elements and Compounds
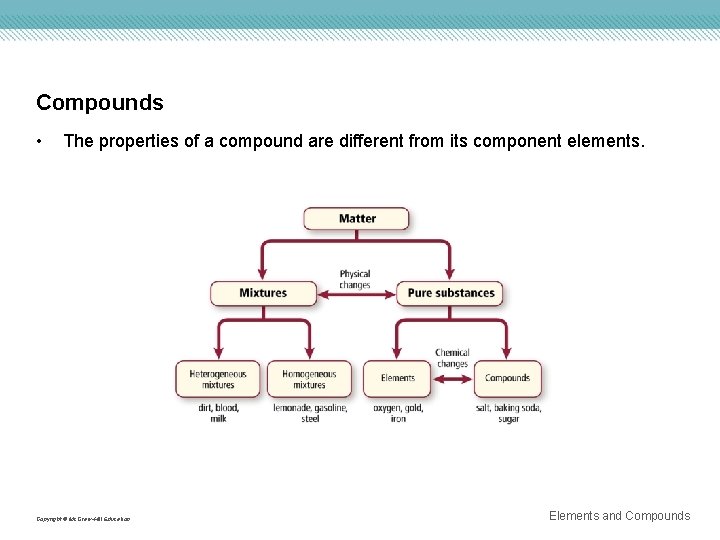
Compounds • The properties of a compound are different from its component elements. Copyright © Mc. Graw-Hill Education Elements and Compounds
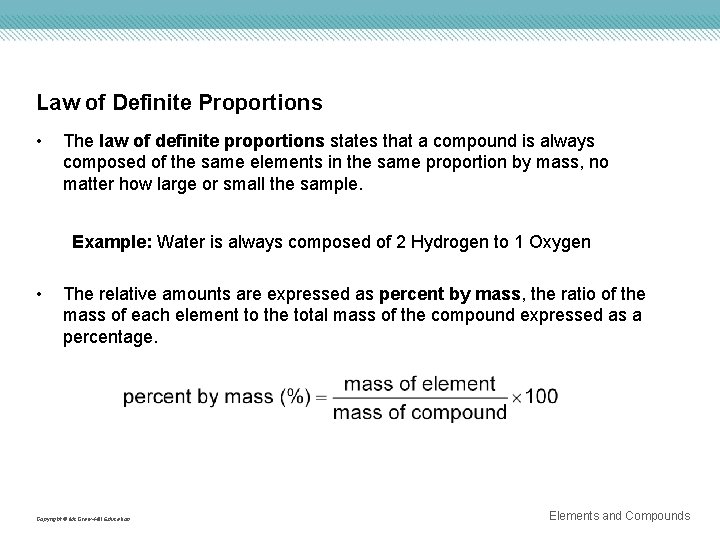
Law of Definite Proportions • The law of definite proportions states that a compound is always composed of the same elements in the same proportion by mass, no matter how large or small the sample. Example: Water is always composed of 2 Hydrogen to 1 Oxygen • The relative amounts are expressed as percent by mass, the ratio of the mass of each element to the total mass of the compound expressed as a percentage. Copyright © Mc. Graw-Hill Education Elements and Compounds
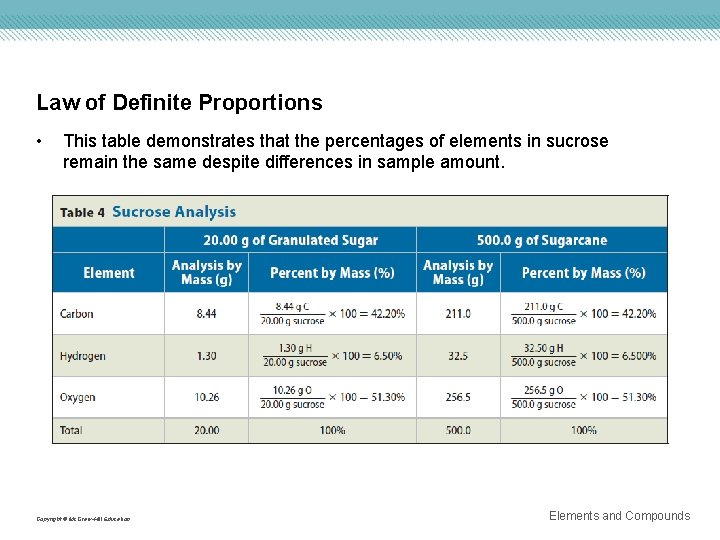
Law of Definite Proportions • This table demonstrates that the percentages of elements in sucrose remain the same despite differences in sample amount. Copyright © Mc. Graw-Hill Education Elements and Compounds
Law of Multiple Proportions • The law of multiple proportions states that when different compounds are formed by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – Example: Peroxide, H 2 O 2, and water, H 2 O • Different compounds formed from the same elements. • Hydrogen mass the same in both compounds but oxygen mass is a 2: 1 ratio in peroxide to water. Copyright © Mc. Graw-Hill Education Elements and Compounds
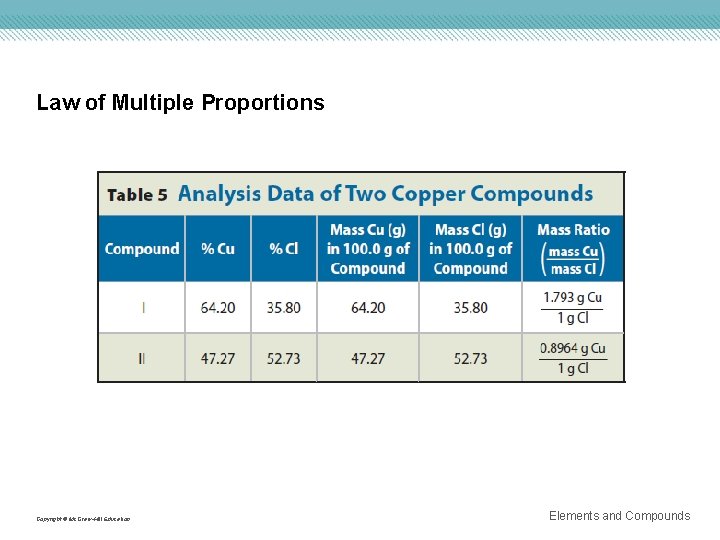
Law of Multiple Proportions Copyright © Mc. Graw-Hill Education Elements and Compounds
Review Essential Questions • What distinguishes elements from compounds? • How is the periodic table organized? • What are the laws of definite and multiple proportions and why are they important? Vocabulary • element • periodic table Copyright © Mc. Graw-Hill Education • compound • law of definite proportions • percent by mass • law of multiple proportions Elements and Compounds
Source: https://slidetodoc.com/section-4-elements-and-compounds-a-compound-is/
0 Response to "Section 3 4 Continued Elements and Compounds"
Post a Comment